Peanut Allergy Clinical Trial Pipeline: Insights into a Growing Landscape with 12+ Companies Advancing Novel Treatments| DelveInsight
Peanut allergy is a growing health concern, especially in children, with rising incidence and increased public awareness of its severity and risks. This heightened awareness among families, schools, and healthcare providers is driving demand for proactive treatments beyond simple avoidance. As a result, the market is witnessing the emergence of innovative therapies like oral immunotherapy, peptides, and mucosal delivery systems aimed at inducing lasting immune tolerance.
New York, USA, Sept. 29, 2025 (GLOBE NEWSWIRE) -- Peanut Allergy Clinical Trial Pipeline: Insights into a Growing Landscape with 12+ Companies Advancing Novel Treatments| DelveInsight
Peanut allergy is a growing health concern, especially in children, with rising incidence and increased public awareness of its severity and risks. This heightened awareness among families, schools, and healthcare providers is driving demand for proactive treatments beyond simple avoidance. As a result, the market is witnessing the emergence of innovative therapies like oral immunotherapy, peptides, and mucosal delivery systems aimed at inducing lasting immune tolerance.
DelveInsight’s 'Peanut Allergy Pipeline Insight 2025' report provides comprehensive global coverage of pipeline peanut allergy therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the peanut allergy pipeline domain.
Key Takeaways from the Peanut Allergy Pipeline Report
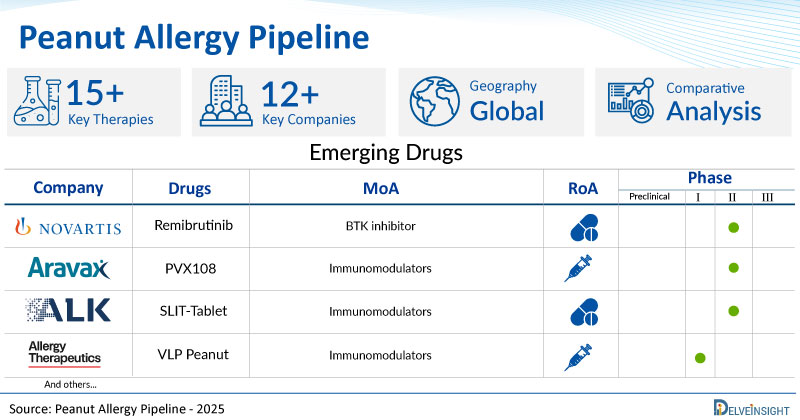
- DelveInsight’s peanut allergy pipeline report depicts a robust space with 12+ active players working to develop 15+ pipeline peanut allergy drugs.
- Key peanut allergy companies such as Allergy Therapeutics, Novartis, Aravax, ALK-Abello, InnoUp Farma, Intrommune Therapeutics, LAPIX Therapeutics, and others are evaluating new peanut allergy drugs to improve the treatment landscape.
- Promising pipeline peanut allergy therapies, such as VLP Peanut, Remibrutinib, PVX 108, Sublingual Immunotherapy (SLIT)-Tablet, INP20, INT301, Food Allergy: Research Program, and others, are in different phases of peanut allergy clinical trials.
- In May 2025, Intrommune Therapeutics announced an Independent Editorial Highlighting breakthrough safety data for novel Peanut Allergy Treatment. The candidate therapy met all its primary and secondary endpoints, achieving exceptional safety and tolerability, with no moderate or severe systemic reactions or anaphylaxis reported in treated participants.
- In March 2025, Allergy Therapeutics announced that its VLP Peanut Phase I/IIa PROTECT trial had entered the final phase of treatment, with healthy volunteers progressing to the highest dose levels and no safety signals observed to date.
- In February 2025, Researchers at Imperial's National Heart & Lung Institute (NHLI) reported encouraging results from the first phase of clinical trials for a new peanut allergy vaccine. The vaccine is being developed in collaboration with industry partner Allergy Therapeutics.
- In October 2024, Aravax announced it had completed patient recruitment into its Phase II study (AVX-201) of its novel, precision therapy PVX108 for the treatment of peanut allergy.
- In January 2024, Aravax announced it had closed its Series B funding round with a total of US USD 42.2m.
Request a sample and discover the recent advances in peanut allergy drugs @ Peanut Allergy Pipeline Report
The peanut allergy pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage peanut allergy drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the peanut allergy clinical trial landscape.
Peanut Allergy Overview
Peanut allergy is among the most prevalent and serious food allergies, especially in children, and is a major public health issue because it can cause potentially fatal anaphylactic reactions. It occurs when the immune system incorrectly identifies peanut proteins as harmful and launches an exaggerated response. Even tiny traces of peanuts can cause reactions ranging from mild skin symptoms to severe respiratory distress and cardiovascular issues. The allergy usually develops in early childhood and often continues throughout life, requiring strict avoidance of peanuts and readiness to use emergency treatments like epinephrine auto-injectors.
Diagnosing peanut allergy involves a detailed medical history and physical examination. Information about the amount and type of food consumed, symptom onset and duration, as well as any relieving factors, is crucial for an accurate diagnosis. A history of eczema is an important risk factor for peanut sensitization. Typical symptoms include skin reactions such as hives, redness, or swelling. More serious signs may involve tingling in the mouth and throat, lip swelling, and difficulty breathing, which can quickly lead to anaphylaxis.
Find out more about peanut allergy drugs @ Peanut Allergy Treatment
A snapshot of the Pipeline Peanut Allergy Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Remibrutinib | Novartis | II | BTK inhibitor | Oral |
| PVX108 | Aravax | II | Immunomodulators | Intradermal |
| Sublingual Immunotherapy (SLIT)-Tablet | ALK-Abello | II | Immunomodulators | Oral |
| VLP Peanut | Allergy Therapeutics | I | Immunomodulators | Subcutaneous |
| INP20 | InnoUp Farma | I/II | NA | Oral |
| INT301 | Intrommune Therapeutics | I | NA | Oral mucosa via brushing |
Learn more about the emerging peanut allergy therapies @ Peanut Allergy Clinical Trials
Peanut Allergy Therapeutics Assessment
The peanut allergy pipeline report proffers an integral view of the emerging peanut allergy therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Peanut Allergy Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal Antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Immunomodulators, BTK inhibitor, Immunostimulants, Immunosuppressants
- Key Peanut Allergy Companies: Allergy Therapeutics, Novartis, Aravax, ALK-Abello, InnoUp Farma, Intrommune Therapeutics, LAPIX Therapeutics, and others.
- Key Peanut Allergy Pipeline Therapies: VLP Peanut, Remibrutinib, PVX 108, Sublingual Immunotherapy (SLIT)-Tablet, INP20, INT301, Food Allergy: Research Program, and others.
Dive deep into rich insights for new peanut allergy treatments, visit @ Peanut Allergy Drugs
Table of Contents
| 1. | Peanut Allergy Pipeline Report Introduction |
| 2. | Peanut Allergy Pipeline Report Executive Summary |
| 3. | Peanut Allergy Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Peanut Allergy Clinical Trial Therapeutics |
| 6. | Peanut Allergy Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Peanut Allergy Pipeline: Late-Stage Products (Phase III) |
| 8. | Peanut Allergy Pipeline: Mid-Stage Products (Phase II) |
| 9. | Peanut Allergy Pipeline: Early-Stage Products (Phase I) |
| 10. | Peanut Allergy Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Peanut Allergy Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Peanut Allergy Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the peanut allergy pipeline therapeutics, reach out @ Peanut Allergy Therapeutics
Related Reports
Peanut Allergy Epidemiology Forecast
Peanut Allergy Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted peanut allergy epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Peanut Allergy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key peanut allergy companies, including DBV Technologies, Novartis, Aravax, ALK-Abello, InnoUp Farma, Intrommune Therapeutics, Stallergenes Greer, Roche, among others.
Food Allergy Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key food allergy companies including Stallergenes Greer, Roche, Novartis, ARS Pharmaceuticals, ALK-Abelló, Alfresa Pharma, DBV Technologies, Aquestive Therapeutics, Bryn Pharma, Aravax, RAPT Therapeutics, Allergy Therapeutics, Regeneron Pharmaceuticals, among others.
Food Allergy Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key food allergy companies, including DBV Technologies, Aravax, Xencor, Novartis AG, Vedanta Biosciences, Alladapt Immunotherapeutics, Intrommune Therapeutics, IgGenix, Lapix Therapeutics, Neovacs, Inimmune, among others.
Lactose Intolerance Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key lactose intolerance companies including CRitter Pharmaceuticals Inc., a2 Milk Company Ltd, VenterPharma, Eurofarma Laboratorios S.A, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
